I’ve recently been reading up on causes of chill haze in whiskey since I’ve started hearing more contradictory things about the phenomena. Chill haze, the tendency of bottled whiskey to become cloudy at lower temperatures, is usually considered mostly harmless, except in the rare case where the haze completely precipitates out, depositing a sediment (AKA floccing).
The haze is explained by the presence of certain compounds in the whiskey which are hydrophobic (i.e. don’t readily dissolve in water) but which do dissolve in ethanol. When the concentration of ethanol in the bottled whiskey is sufficiently high, these compounds remain in solution even when the whiskey is cooled. But when the ethanol concentration is lowered (i.e. the bottling proof drops below about 100) these compounds are more likely to come out of solution and form a haze.
The haze can be remediated by the process known as chill filtration, performed before bottling. This involves reducing the temperature of the whiskey down to the point where the haze forms and then lightly filtering it to remove the particles which drop out of solution. The bottled whiskey will no longer exhibit the haze at lower temperatures.
Which church do you attend?
There are two common schools of belief (or perhaps ‘churches’ is a better term) on chill filtering. One school declares that chill filtering is a harmless process and that what’s removed has no impact on flavor, only color and possibly the texture (mouthfeel) of the whiskey is affected. Some adherents will go as far as to say the whiskey can actually be improved by the filtering process. The other school strongly believes that chill filtering is destructive and results in an unavoidable attenuation of aroma and flavor in the whiskey.
It seemed unlikely that both of these assertions could be true so I decided to look further into the matter.
Not one but two causes!
After some investigation it would seem that, surprisingly enough, the assertions of both schools hold true because there are two distinct causes for the chill haze phenomena. And both may be present at the same time in a whiskey.
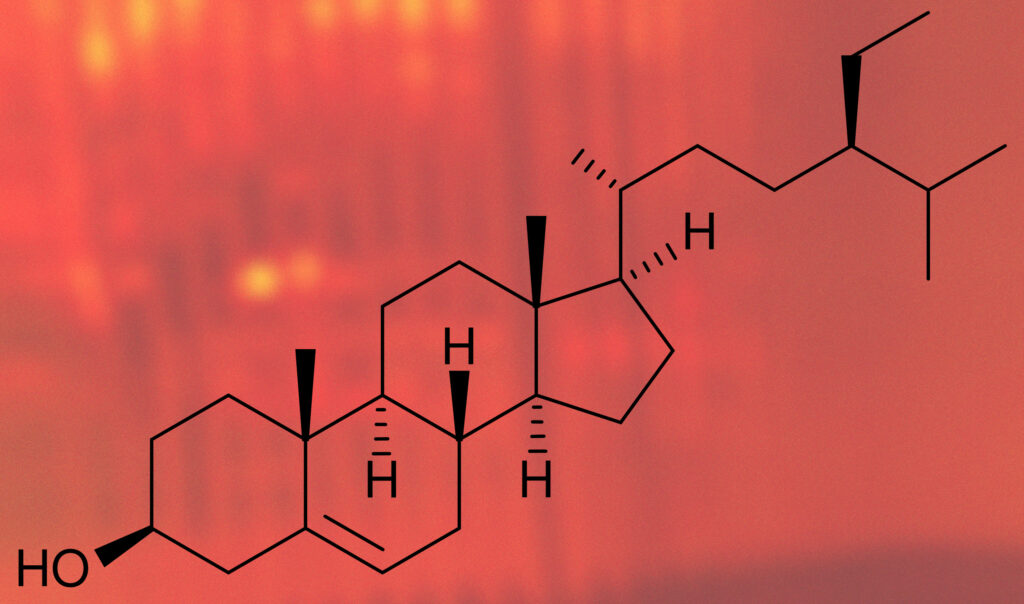
The first cause is a class of compounds known as phytosterols, specifically β-Sitosterol, stigmasterol, and camposterol. These compounds apparently come directly from oak, i.e. are not formed during fermentation or distillation, and are merely ‘washed out’ of the wood during maturation. Sterols have no appreciable aroma or taste but they can contribute to color and texture of the whiskey. Removing these via chill filtering should in theory result in whiskey losing some body/mouthfeel but not much else.
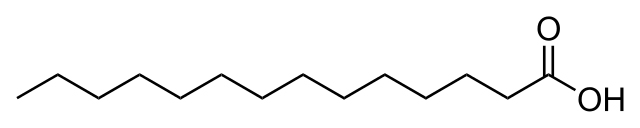
The second cause is attributed to the presence of two different esters in the whiskey, ethyl hexadecanoate and ethyl myristate (AKA ethyl tetradecanoate). These are formed by the interaction of myristic or palmitic acid (both long chain fatty acids or LCFAs) and ethanol. Esters such as these are likely formed in the heat of distillation and/or during maturation. These two esters present distinct organoleptic qualities, usually described as waxy, fruity, and/or creamy aromas. Removing these esters would very likely result in the attenuation of aroma and taste of the whiskey.
In a final twist, phytosterols might not be a significant cause of chill haze in Scotch whisky. Because these come from a finite source, the barrel used for maturation, and because Bourbon and rye are aged exclusively in new barrels, a lot of the phytosterols in the oak get washed out during maturation. When the barrels are reused for aging Scotch whisky, there’s much less of the phytosterols left to wash out. So phytosterols are not considered to be a significant contributor to chill haze in Scotch whisky.
Now, unless there’s a way to very selectively chill filter these compounds out, I would have to conclude that the second school (‘Chill filtering is evil!’) is right and the process will change the organoleptic qualities of the whiskey. But then again, it’s also reasonable to wonder how many whiskies contain ethyl hexadecanoate and/or ethyl myristate and in amounts significant enough for chill filtering to affect their organoleptic qualities. Perhaps they are not that common? [1]
And finally perhaps there’s a way to have one’s cake and eat it too? If the filtering can be ‘dialed in’ to remove the phytosterols and leave the two more interesting esters behind, then you’ve at least split some of the difference between the two camps. I have no idea of this is possible but it makes me wonder if this is what Michter’s claims to be doing when they speak of different chill filtering ‘protocols’ for their whiskies. [2]
—
Notes:
[1] I didn’t feel I had the science knowledge to fully explore in this direction but wanted to mention the possibility anyway. First, not all yeasts are likely to make the necessary precursors, myristic and palmitic acids. Second, both have rather high boiling points. So even if present in the beer, whether they make it through distillation may depend on the type of distillation and (in the case of potstills) where cuts for heads and tails are made as well as the geometry of the dome and lyne arm. And finally conditions under which the two associated esters form also have to occur at some point during maturation. So…as I speculated, there may be whiskies that simply don’t contain these.
[2] This sounds good but intuitively this seems like it would require that the temp at which the phytosterols come out of solution to form a haze be sufficiently higher than the temp at which ethyl hexadecanoate and/or ethyl myristate do the same. This would allow the former to be removed while the latter remains in solution. Of course, the concentration of ethanol during filtration is another variable that could be controlled, i.e. the filtration could be done before diluting to final bottling proof, thereby affecting the temp at which these compounds come out of solution. But Michter’s rather low barrel exit proof (around 107 – 112 as per their website) doesn’t seem like it would provide much range in which play with this, if it would even matter.



